This settlement among the United States, the States, and pharmaceutical companies Mallinckrodt plc and its subsidiary Mallinckrodt ARD LLC resolved allegations that Mallinckrodt violated the federal and state False Claims Acts by knowingly underpaying Medicaid rebates for its high-priced drug Acthar. The total settlement amount is $233,707,865 (plus interest). The United States will receive $123,642,146, and States will receive $110,065,718.
Mallinckrodt knowingly misreported Acthar’s base Average Manufacturer Price (“base AMP”) from January 2013 through June 2020. By doing so, it reduced the rebates it paid to the Medicaid Drug Rebate Program (MDRP) by approximately $650 million. Mallinckrodt had increased Acthar’s price from approximately $50 per vial in 2001 to almost $40,000 per vial.
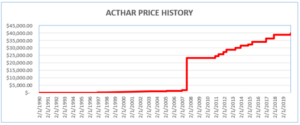
Where a drug’s price is increased above the rate of inflation, manufacturers must pay an additional rebate. To avoid meeting its increased rebate obligations, Mallinckrodt began reporting Acthar’s base AMP as if it had been approved in 2010 (after the enormous price increases). Acthar, however, had been approved in 1952.
Our client, James Landolt, served as Mallinckrodt’s Director of Internal Controls, Gross to Net Accounting and Government Reporting from November 2015 until July 2017. In that position, he learned that Mallinckrodt had been misreporting the base AMP for Acthar and had underpaid the MDRP by hundreds of millions of dollars.
Mr. Landolt resigned from Mallinckrodt in 2017 and filed a qui tam action in 2018 alleging that Mallinckrodt’s knowing failure to pay correct rebates for Acthar violated federal and state False Claims Acts. In March 2020, the United States intervened in his lawsuit. In June 2020, twenty-eight states, the District of Columbia, and Puerto Rico also intervened.
While the False Claims Act was still under seal, Mallinckrodt sued the Center for Medicare and Medicaid Services (CMS) in federal court in the District of Columbia. Mallinckrodt sought a ruling that it was correctly reporting Acthar’s base AMP and did not have to comply with instructions from CMS to correct its reporting and pay what it owed. In March 2020, the District Court rejected Mallinckrodt’s argument. Two months later, it rejected Mallinckrodt’s motion for reconsideration and for a preliminary injunction.
In October 2020, Mallinckrodt filed for bankruptcy, which stayed the pending False Claims Act case. On March 2, 2022, the bankruptcy court confirmed Mallinckrodt’s plan of reorganization, which included this $234 million settlement.
As part of the settlement, Mallinckrodt entered into a five-year Corporate Integrity Agreement with HHS-OIG that requires, among other things, an independent review organization to annually review multiple aspects of the company’s practices relating to the Medicaid Drug Rebate Program. Mallinckrodt began reporting the correct base AMP for Acthar in June 2020 after losing its case in United States Court in D.C.
Mr. Landolt will receive a 20% share of amounts paid under the federal and state False Claims Acts. Whistleblowers like Mr. Landolt are critical in the fight against fraud. Under the False Claims Act, a private citizen-relator who suspects or knows of fraud against the government can file a sealed complaint on behalf of the government. In successful cases, the relator is entitled to a share of the government’s recovery.