March 28, 2022
Law360 recently reported on the success of our whistleblower client in a False Claims Act case against Mallinckrodt. Earlier this month, the Department of Justice announced that Mallinckrodt had agreed to pay nearly $234 million to resolve allegations that it had knowingly underpaid Medicaid rebates on its drug Acthar. For his role in bringing this case, our client will receive over $40 million from the settlement.
Mallinckrodt should have been paying higher rebates because the company had raised the price of Acthar dramatically. Acthar’s price increased from approximately $50 per vial in 2001 to $40,000 per vial today. 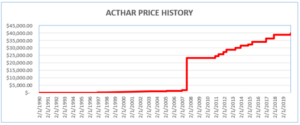
In 2018, our client, James Landolt, brought a qui tam case against Mallinckrodt under the False Claims Act. He alleged that Mallinckrodt had cheated the Medicaid program by knowingly reporting an incorrect base date Average Manufacturer Price for Acthar. By misreporting Acthar’s base AMP, Mallinckrodt avoided paying hundreds of millions of dollars of rebates owed to Medicaid. Details about the case and settlement can be found here.
The Law360 article focuses on our client’s role as a whistleblower. Mr. Landolt’s position at Mallinckrodt included oversight of the group responsible for reporting pricing information to the Medicaid Drug Rebate Program. The Center for Medicare & Medicaid Services notified Mallinckrodt in 2016 that it had been misreporting Acthar’s base AMP. Although CMS instructed Mallinckrodt to correct its data reporting, it did not do so. As the Law360 article notes:
[A]fter Mallinckrodt received a CMS follow-up letter in March 2017 and did not correct Acthar’s base average manufacturer price, Landolt “lacked confidence that its senior leadership would address the issue to his satisfaction,” said Linda Severin, one of Landolt’s attorneys at the Whistleblower Law Collaborative and an ex-assistant U.S. attorney in the Southern District of New York.
After resigning from Mallinckrodt, Mr. Landolt contacted the Whistleblower Law Collaborative. The article quotes Linda Severin describing the firm’s response:
“When Mr. Landolt came to us with his concerns, we immediately recognized the significance of this matter because our firm had handled the Mylan EpiPen rebate case,” Severin said, referring to a 2016 case where Mylan paid $465 million for misclassifying the emergency allergy EpiPen shot as a generic drug to avoid paying the higher rebates. “We were able quickly to bring his concerns to the [DOJ] so the authorities could take action.”
The article also quotes Suzanne Durrell about the importance both of the Medicaid Drug Rebate Program and of strong enforcement efforts, such as those by federal and state authorities in this case:
“Congress enacted the Medicaid Drug Rebate Program to protect Medicaid programs from excessive price increases for drugs,” said Suzanne Durrell of the Whistleblower Law Collaborative, who also represented Landolt and is the ex-chief of the civil division of the Boston U.S. attorney’s office.
“State Medicaid programs are financially strapped and serve the poorest people in the country,” Durrell said in an emailed statement to Law360. “We feel it’s vitally important that the Medicaid programs not be defrauded and that drug companies live up to their reporting obligations. When companies fail to fulfill those obligations, it’s essential to hold them accountable.”
The Whistleblower Law Collaborative LLC, based in Boston, is a top whistleblower law firm that devotes its practice entirely to representing clients in bringing actions under the federal and state False Claims Acts and other whistleblower programs. For more information, contact us at 617.366.2800.
How A Drug Co.'s Misreporting Earned A Whistleblower $40M