March 7, 2022
On March 7, 2022, the United States, individual states, the District of Columbia, and Puerto Rico announced a False Claims Act settlement related to rebate fraud allegations brought by our client, James Landolt, against Mallinckrodt plc and its subsidiary Mallinckrodt ARD LLC. The case alleged that for several years, Mallinckrodt knowingly underpaid Medicaid rebates for its high-priced drug Acthar. Mallinckrodt will pay a total of $233,707,865 (plus interest) in eight installments. The United States will receive $123,642,146, and the Medicaid Participating States will receive $110,065,718. In addition, Mallinckrodt has entered into a Corporate Integrity Agreement.
We have previously described the Medicaid Drug Rebate Program (MDRP). Briefly, the MDRP requires manufacturers to pay quarterly rebates to state Medicaid programs. Companies pay rebates based, in part, on a drug’s reported Average Manufacturer Price (AMP). In addition, if a company increases a drug’s price above the rate of inflation, its rebate obligation increases. This additional inflationary rebate helps to offset costs to the Medicaid program from excessive price increases. The inflationary rebate is based on the drug’s price when it first was marketed or 1990 (whichever is later). This is known as the drug’s “base AMP.”
Mr. Landolt served as Mallinckrodt’s Director of Internal Controls, Gross to Net Accounting and Government Reporting from November 2015 until July 2017. In that position, he learned that Mallinckrodt had been misreporting the base AMP for Acthar, which reduced the rebates Mallinckrodt paid to the MDRP by hundreds of millions of dollars. The Centers for Medicare & Medicaid Services (CMS) notified Mallinckrodt in 2016 that it had been reporting an incorrect base AMP for Acthar and directed the company to correct its data reporting. Mallinckrodt, however, continued to report an improperly high base AMP and cheated taxpayers out of approximately $650 million in rebates from 2013 until 2020.
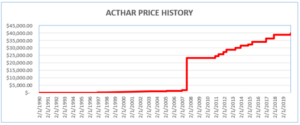
Acthar was first approved by the FDA in 1952. In 2001, it cost approximately $50 per 5 ml vial. Since then, Mallinckrodt increased the price of Acthar to approximately $40,000 per vial. It is now one of the most expensive medications in the United States. Mallinckrodt should have reported Acthar’s base AMP based on its price in 1990. Instead, starting in 2013, Mallinckrodt began reporting data to the MDRP as if Acthar had been approved in 2010 (after the enormous price increases).
Mr. Landolt resigned from Mallinckrodt in 2017 and filed a qui tam action in 2018 alleging that Mallinckrodt’s knowing failure to pay correct rebates for Acthar violated federal and state False Claims Acts. In March 2020, the United States intervened in his lawsuit. In June 2020, twenty-eight states, the District of Columbia, and Puerto Rico also intervened. The case is United States ex rel. James Landolt v. Mallinckrodt ARD LLC, 1:18-cv-11931-PBS (D. Mass.).
In October 2020, while the case was pending, Mallinckrodt filed for bankruptcy. On March 2, the bankruptcy court issued an order confirming Mallinckrodt’s reorganization plan. Because of Mallinckrodt’s bankruptcy, federal and state governments were not able to recoup the full amount of the unpaid Acthar rebates. However, Mallinckrodt finally began reporting Acthar rebates properly in 2020.
Mr. Landolt will receive a 20% share of the settlement amount. In reflecting on his experience, he noted: “It is easy to feel powerless in these types of situations. But individuals have a voice and the power to do something about wrongdoing, thanks to whistleblower laws like the False Claims Act.” Mr. Landolt added, “I am very grateful to my attorneys Linda Severin and Suzanne Durrell for their legal guidance and moral support throughout the process. I also deeply appreciate the hard work and commitment that government attorneys and investigators brought to this case.”
It was an honor to represent Mr. Landolt in this case.
Mr. Landolt’s moral compass would not allow him to stand by and do nothing as Mallinckrodt continued to underpay its Medicaid rebate obligations by hundreds of millions of dollars.
We are very grateful for the outstanding efforts of the government on this case. Government attorneys fought this case on multiple fronts – in Massachusetts, where our client filed his qui tam case; in D.C., where Mallinckrodt unsuccessfully sued CMS to avoid having to report Acthar’s correct base AMP; and, finally, in bankruptcy court in Delaware. Department of Justice and state Attorneys General Office attorneys worked enormously hard to hold Mallinckrodt accountable and secure this victory for taxpayers. We especially commend the efforts of Assistant U.S. Attorney Evan Panich and former Assistant U.S. Attorney Gregg Shapiro of the District of Massachusetts and Department of Justice Civil Fraud Trial Attorneys Augustine Ripa and Michael Hoffman, as well as Katie Wilson of the Wisconsin Attorney General’s Office and C. Ian Garland of the Florida Attorney General’s Office, who coordinated the efforts of the State Attorneys General.
The United States’ press releases are available here (DOJ) and here (District of Massachusetts). Additional documents pertaining to this case, including the settlement agreement, can be viewed here. In announcing today’s settlement, United States Attorney Rachael S. Rollins stated:
Today’s settlement vindicates the interests of the American taxpayer by ensuring that no pharmaceutical manufacturer can illegally boost its profits at the expense of state Medicaid programs, and the people and families those programs serve. This company unlawfully siphoned money out of the Medicaid program which poor people depend on for their medical care.
Joseph R. Bonavolonta, Special Agent in Charge of the Federal Bureau of Investigation, Boston Division, noted:
This settlement resolves allegations that Mallinckrodt cheated the Medicaid program, and ultimately taxpayers, out of hundreds of millions of dollars, by exploiting a system that was set up to keep a check on rising drug prices to ensure that our most vulnerable citizens are able to receive medical treatment.
This is the second major pharmaceutical fraud settlement for the Whistleblower Law Collaborative in a Medicaid rebate fraud case. In 2017, Mylan paid $465 million for misclassifying EpiPen® as a generic drug to avoid paying the higher rebates owed on brand name drugs.
The Whistleblower Law Collaborative LLC, based in Boston, is a top whistleblower law firm that devotes its practice entirely to representing clients in bringing actions under the federal and state False Claims Acts and other whistleblower programs. Under the False Claims Act, a private citizen (known as a “relator”) who suspects or knows of fraud against the government can act as a whistleblower and file a sealed complaint on behalf of the government. A relator in a successful case is entitled to a share of the government’s recovery.
For more information, contact us at 617.366.2800.